.png) Agenzia Italiana del Farmaco
Agenzia Italiana del Farmaco
Horizon scanning
Horizon Scanning is generally defined as the systematic identification of new, emerging or outdated health technologies that have a potential impact on health, health services and society. In the context of AIFA, this activity has a strategic function because it allows the early identification and evaluation of new medicines and new therapeutic indications of medicinal products already on the market that, once marketed, could have a significant clinical and economic impact on the National Health Service.
The main objective is to support the decision-making processes of the Agency through the production of information documents useful to plan ahead the introduction of innovative medicines and to promote a correct and transparent allocation of the economic resources of the National Health Service.
Finally, Horizon Scanning makes it possible to provide early information to healthcare professionals, patients, citizens and associations on potentially promising therapeutic strategies to come.
The phases of Horizon Scanning in AIFA
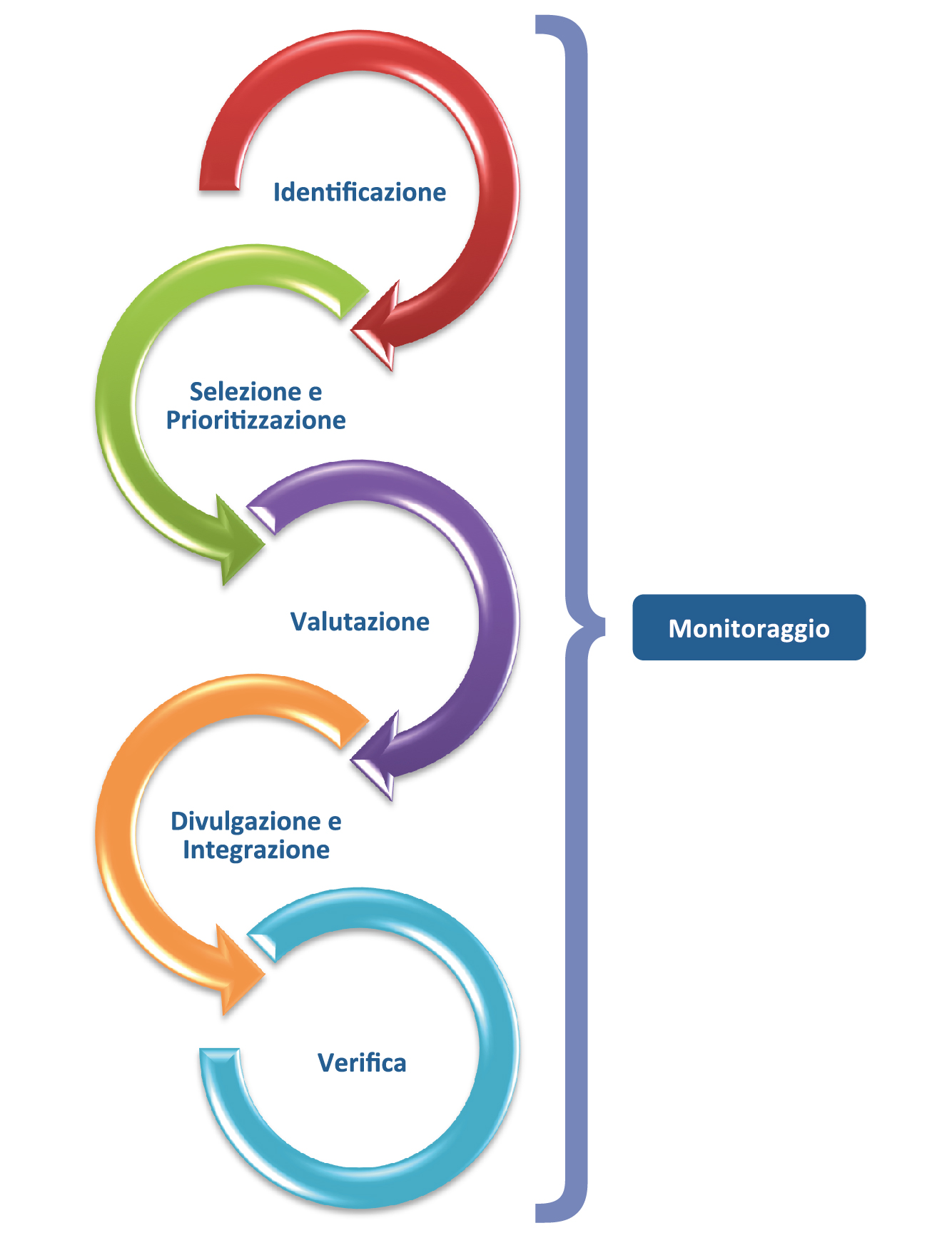 AIFA’s Horizon Scanning activity consists of 3 consecutive phases (identification, selection and prioritisation, evaluation) to which 2 further phases are added with different frequency (dissemination and integration in the decision-making process of the generated information, periodic verification of the data produced).
AIFA’s Horizon Scanning activity consists of 3 consecutive phases (identification, selection and prioritisation, evaluation) to which 2 further phases are added with different frequency (dissemination and integration in the decision-making process of the generated information, periodic verification of the data produced).
The Horizon Scanning reports support the various activities of the Agency’s Offices, the Support and Coordination Secretariats and the AIFA Technical and Advisory Committees.
All phases of the Horizon Scanning activity are continuously monitored through the use of appropriate indicators (Key Performance Indicators).
The identification phase consists essentially of the systematic collection of information on new medicinal products and new therapeutic indications of medicinal products already on the market arriving within 12-36 months.
Different sources of information are used to collect this information.
The identified medicinal products are subject to selection criteria in order to exclude certain categories of medicinal products with known clinical and/or economic impact and subsequently to prioritisation criteria for the assessment of the presumed clinical and economic impact. For potentially more interesting medicinal products from a scientific point of view, the expected clinical-therapeutic aspect is studied in depth.







