.png) Agenzia Italiana del Farmaco
Agenzia Italiana del Farmaco
Economic evaluations
Economic evaluations are a tool for defining the value of a medicinal product in terms of cost-opportunity, from the point of view of the patient, the NHS and the society as a whole.
The definition of "value" is very broad, multidimensional and includes concepts from different disciplines, beyond the economic one. Specifically, for the economic evaluations concerning new medicinal products, innovative or not, the value is given by the marginal utility that the patient, the NHS and/or the society can obtain from its acquisition. In this regard, the measurement of the years of life gained in full quality of life (QALY – quality-adjusted life years) is widely applied to medicinal products in different regulatory contexts, although with the awareness that it is not able to grasp all the elements that contribute to value.
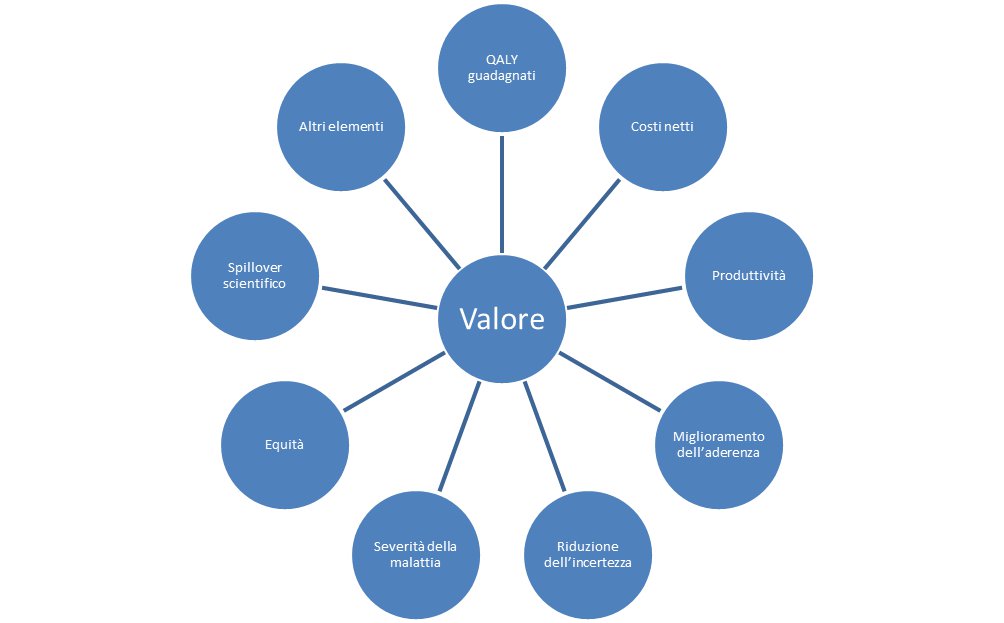
The World Health Organization defines "pharmacoeconomic analysis" as the set of analysis methodologies aimed at identifying, measuring and evaluating the costs and related consequences (benefits/outcomes) of two or more therapeutic alternatives. From a technical point of view, cost analyses (including budget impact analyses), cost-benefit, cost-effectiveness, cost-utility and cost-minimisation analyses fall within the pharmacoeconomic analyses.
Although the cost-effectiveness criterion has been introduced with reference to the negotiation of the price and eligibility for reimbursement of medicinal products to be charged to the NHS since 1997 (CIPE Resolution No. 5 of 30 January 1997), and then subsequently referred to also by CIPE Resolution No. 3 of 1 February 2001, it is only after the implementation of the Regulation for the organisation, functioning and organisation of the AIFA staff, definitively adopted with Resolution No. 8 of 8 April 2016, that economic evaluations have had a practical integration in the assessment procedures by AIFA in support of the appraisal by the CTS/CPR committees.
Organisation of the economic evaluation activity
The economic evaluation activity in AIFA consists in evaluating the cost-effectiveness and sustainability profile of medicinal products as part of the process of defining the eligibility for reimbursement and the price charged to the NHS. The assessment of the economic and financial impact of medicinal products is an essential component of the Health Technology Assessment process (EUnetHTA, 2016), a systematic, multidimensional and multidisciplinary approach used at international level for the assessment of health technologies.
The process of defining the eligibility for reimbursement and price of medicinal products starts with the presentation of the reimbursement and price dossier by the pharmaceutical company and ends with the resolution of the AIFA Board of Directors and subsequent publication in the Official Journal, after obtaining the opinion of the Technical-Scientific Committee (CTS) and the Price and Reimbursement Committee (CPR). The price is determined through negotiation between AIFA and the pharmaceutical companies (Law No. 326 of 24 November 2003).
According to the Ministerial Decree 2 August 2019, pharmaceutical companies are required to submit a P&R dossier in line with the provisions of the AIFA Guideline to the submission of applications published on the website of the Agency.
The submission of budget impact analyses and economic evaluations is expressly requested only for new medicinal products, orphan medicines and/or for new therapeutic indications of patented products, which fall within the scope of TN1 procedures. Technical and methodological requirements of such analyses are detailed in Section E and Appendix 2 of the AIFA Guideline.
Based on pharmacoeconomic analyses submitted by companies, AIFA prepares opinions of a non-mandatory and non-binding nature for the CPR, which constitute a support tool in the decision-making process of defining the price and eligibility for reimbursement of a medicinal product.
The preliminary investigation includes the following steps:
- critical evaluation of the pharmacoeconomic studies submitted by pharmaceutical companies within the Price and Reimbursement Dossier;
- revision of the pharmacoeconomic model where transmitted by the company in an open and editable format;
- literature review for the identification of further published pharmacoeconomic studies relating to the national or international context;
- identification of recommendations and decisions taken in other countries concerning the medicinal product under application;
- analysis of treatment costs compared to therapeutic alternatives;
- economic-financial impact assessment.
The critical evaluation of the pharmacoeconomic studies submitted by the pharmaceutical companies is carried out by verifying compliance with the standards developed by the ISPOR Task Force for cost-effectiveness and budget impact analyses (Husereau et al., 2013; Sullivan et al., 2014); moreover, for the evaluation of the quality and robustness of the studies, AIFA makes use of internationally recognized and validated tools, such as the check-lists of Drummond et al. 2015 and Philips et al., 2004.
The final opinion is sent to the HTA Secretariat for discussion in plenary and subsequently to the members of the Committees within the preliminary documentation.
Role of economic evaluations on pricing and reimbursements decisions
The first study on the role of economic evaluations in the pricing and reimbursement of medicines in Italy has been published in the international journal Pharmacoeconomics. The article, entitled “Role of Economic Evaluations on Pricing of Medicines Reimbursed by the Italian National Health Service” is the result of an analysis carried out by the Health Economic Evaluation Office at AIFA, with the commitment and supervision of Prof. Andrea Manca of the Centre for Health Economics at the University of York.
The study shows that the result of cost-effectiveness analyses submitted to AIFA by pharmaceutical companies is one of the factors that predicts the final outcome of price negotiation. Such result suggests that in Italy the setting of medicine prices takes into account the value of medicines for patients and the National Health Service.
Greater clarity on the definition of “value” and greater use of economic evaluations in pricing and reimbursement processes can contribute to ensure the best use of public resources for national healthcare. The submission of pharmacoeconomic analyses, explicitly required by the new AIFA guidelines for new medicinal products, orphan medicines and new therapeutic indications, represents the first step in this direction.
Normative references
- Ministerial Decree 2 August 2019. Criteria and methods with which the Italian Medicines Agency determines, through negotiation, the prices of medicines reimbursed by the National Health Service (Official Journal n. 185 24 July 2020);
- Act DG/1372/2020. Guidelines and procedures for submitting to AIFA applications for reimbursement and pricing by pharmaceutical companies;
- Article 48, paragraph 5, of the Law Decree no. 269 of 30 September 2003, converted, with amendments, into Law no. 326 of 24 November 2003, which identifies the tasks of AIFA with respect to the marketing of medicines charged to the NHS;
- Article 48, paragraph 33, of the Law Decree no. 269 of 30 September 2003, converted, with amendments, into Law no. 326 of 24 November 2003, which establishes the criteria and procedures for the negotiation of medicines to be charged to the NHS with the pharmaceutical companies;
- CIPE Resolution 1 February 2001, no. 3, published in the Official Journal. no. 73 of 28 March 2001, containing: "Identification of criteria for negotiating the price of medicinal products";
- Regulation for the organisation, functioning and staff-organisation of the Italian Medicines Agency (AIFA Resolution 8 April 2016, no. 8).
Bibliographical references
- Carletto, A., Zanuzzi, M., Sammarco, A., & Russo, P. Quality of health economic evaluations submitted to the Italian Medicines Agency: current state and future actions. International Journal of Technology Assessment in Health Care, 1-9.
- Drummond, M. F., Sculpher, M. J., Claxton, K., Stoddart, G. L., & Torrance, G. W. (2015). Methods for the economic evaluation of health care programmes. Oxford university press.
- EUnetHTA Joint Action 2, Work Package 8. HTA Core Model ® version 3.0 (Pdf); 2016. Available from www.htacoremodel.info/BrowseModel.aspx.
- Husereau, D., Drummond, M., Petrou, S., Carswell, C., Moher, D., Greenberg, D., ... & Loder, E. (2013). Consolidated health economic evaluation reporting standards (CHEERS) statement. Cost Effectiveness and Resource Allocation, 11(1), 6.
- Lakdawalla, D. N., Doshi, J. A., Garrison Jr, L. P., Phelps, C. E., Basu, A., & Danzon, P. M. (2018). Defining elements of value in health care—a health economics approach: an ISPOR Special Task Force report [3]. Value in Health, 21(2), 131-139.
- Philips, Z., Ginnelly, L., Sculpher, M., Claxton, K., Golder, S., Riemsma, R., ... & Glanville, J. (2004). Review of guidelines for good practice in decision-analytic modelling in health technology assessment. In NIHR Health Technology Assessment programme: Executive Summaries. NIHR Journals Library.
- Sullivan, S. D., Mauskopf, J. A., Augustovski, F., Caro, J. J., Lee, K. M., Minchin, M., ... & Shau, W. Y. (2014). Budget impact analysis—principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value in health, 17(1), 5-14.







